Menopause and Hormone Replacement Therapy
This formulary page covers diagnosis and management of menopause following NICE guideline [NG23] Menopause: diagnosis and management.
Prescribing Guidelines by Clinical Area
The guidance aims to support in the diagnosis and management of menopause including people with premature ovarian insufficiency to support GPs so they can optimise their patient’s treatment choices with them.
Please expand the topics below to see more detail. There are several resources also under Resources below, some of these will be useful to use with patients, but they may also support you in CPD. The HRT formulary is linked, however specific detail is shared as treatment pathways in each of the further pages under transdermal, oral or topical options.
Menopause Guidance
BMS Guidance, Stock Availability and Safety
Epilepsy and HRT? - Consider interactions - access BNF interactions
See the SPS supply availability page for current stocks- Log in to access this page
NHSBSA Serious Shortage Protocols for current serious shortage protocols
Learn about the Somerset NHS Menopause Service.
BMS Consensus Statements - British Menopause Society including:
- The British Menopause Society (BMS) have provided BMS Tools for Clinicians:
- *NEW* Measurement of serum estradiol in the menopause transition - BMS July 2025
- What is the menopause?
- Menopause practice standards
- NICE: Menopause, Diagnosis and Management – from Guideline to Practice Guideline Summary
- Menopause: Guidance for Practice Top Ten Tips
- HRT Guide
- HRT – Practical prescribing
- HRT preparations and equivalent alternatives -Useful when looking at stock equivalents during supply issues.
- Progestogens and endometrial protection
- Management of menopause for women with cardiovascular disease
- HRT after myocardial infarction
- Fast Facts: HRT and breast cancer risk
- Prescribable alternatives to HRT
- Testosterone replacement in menopause
- Cognitive Behaviour Therapy (CBT) for Menopausal Symptoms
- Menopause: Nutrition and Weight Gain
- Menopause: Nutrition and Weight Gain Top Ten Tips
- Surgical Menopause
- Induced menopause in women with endometriosis
- Migraine and HRT
- HIV and the menopause
- Menopause in ethnic minority women
- Menopause and the Workplace Guidance: what to consider
*Under Review* December 2024
NHS Somerset netFormulary: Hormone Replacement Therapy
British Menopause Society – Tools for clinicians
MHRA Summary of HRT risks and benefits
[NG23] Menopause: diagnosis and management
British Menopause Society: HRT Guide July 2020
NHS Employers: Menopause and the workplace
The Somerset Emotional Wellbeing Podcast: Demystifying the Menopause – Hosted by Dr Andrew Tresidder featuring Dr Kathryn Patrick
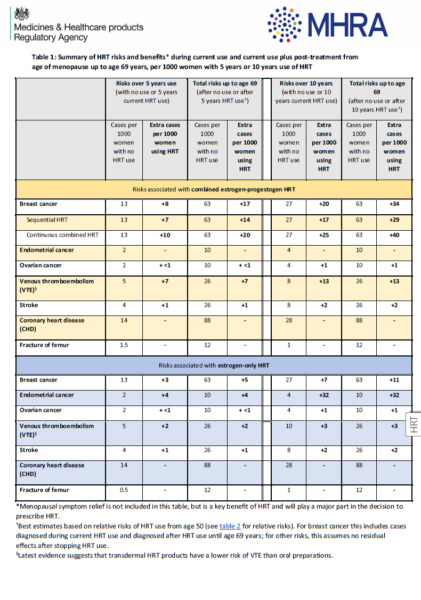
HRT and the likelihood of some medical conditions A discussion aid for healthcare professionals and patients from NICE guideline NG23- Menopause: identification and management updated 07 November 2024.
Venous thromboembolism
•the risk of venous thromboembolism (VTE) is increased by oral HRT compared with baseline population risk
•the risk of VTE associated with HRT is greater for oral than transdermal preparations
•the risk associated with transdermal HRT given at standard therapeutic doses is no greater than baseline population risk.
•Consider transdermal rather than oral HRT for menopausal people who are at increased risk of VTE, including those with a BMI over 30 kg/m2
•Consider referring menopausal people at high risk of VTE to a haematologist for assessment before considering HRT.
Cardiovascular disease
•HRT does not increase cardiovascular disease risk when started under 60 years
•HRT does not affect the risk of dying from cardiovascular disease
•the presence of cardiovascular risk factors is not a contraindication to HRT as long as they are optimally managed
•HRT with oestrogen alone is associated with no, or reduced, risk of coronary heart disease
•HRT with oestrogen and progestogen is associated with little or no increase in the risk of coronary heart disease
•Explain that taking oral (but not transdermal) oestrogen is associated with a small increase in the risk of stroke and the baseline population risk of stroke in women aged under 60 years is very low.
Osteoporosis
Give advice on bone health and discuss these issues at review appointments.
Explain that the baseline population risk of fragility fracture for women around menopausal age in the UK is low and varies from one woman to another.
Explain to women that their risk of fragility fracture is decreased while taking HRT and that this benefit:
•is maintained during treatment but decreases once treatment stops
•may continue for longer in women who take HRT for longer.
Review patients for risk of osteoporosis and fragility fractures in line with NICE Clinical Knowledge Summary: Osteoporosis – Prevention of fragility fractures: Assessment. Check FRAX score and consider bisphosphonate treatment if indicated. See formulary chapter 6.6.
Ensure patients are taking vitamin D as self-care in line with [PH56] Vitamin D: supplement use in specific population groups and ensure adequate dietary intake of calcium.
People with or at High Risk of, Breast Cancer
Offer menopausal women and people with, or at high risk of breast cancer:
-Information on all available treatment options
-Information that the SSRIs paroxetine, fluoxetine, or sertraline should not be offered to people with breast cancer who are taking tamoxifen
-Referral to a healthcare professional with expertise in menopause. See the Somerset NHS Menopause Service for referral criteria and information.
Using the MHRA risk table, explain to people around the age of natural menopause that:
-the baseline risk of breast cancer for people around menopausal age varies from one person to another according to the presence of underlying risk factors
-HRT with oestrogen alone is associated with little or no change in the risk of breast cancer
-HRT with oestrogen and progestogen can be associated with an increase in the risk of breast cancer
-any increase in the risk of breast cancer is related to treatment duration and reduces after stopping HRT.
Menopausal Symptoms with Breast Cancer
-Information and counselling should be offered about the possibility of early menopause and menopausal symptoms associated with breast cancer treatment.
-Stop systemic hormone replacement therapy (HRT) in people who are diagnosed with breast cancer.
-Do not routinely offer HRT (including oestrogen/progestogen combination) routinely to people with menopausal symptoms and a history of breast cancer.
-In exceptional circumstances, offer HRT to people with severe menopausal symptoms and with whom the associated risks have been discussed in conjunction with the oncologist (unlicensed use- HRT is contraindicated with a history of breast cancer). ALL alternatives should be trialled before taking this step, the patient should be fully aware of the associated risks of this treatment option, with input from a menopause specialist.
-Consider selective serotonin reuptake inhibitor (SSRI) antidepressants for people with breast cancer for relieving menopausal symptoms, particularly hot flushes, but not for those taking tamoxifen. However, there is no clear evidence for use of SSRIs or SNRIs. The BMS guidance on prescribable alternatives to HRT may be useful.
Do not offer soy (isoflavone), red clover, black cohosh, vitamin E or magnetic devices to treat menopausal symptoms in people with breast cancer.
For those with a family history of breast cancer who are considering taking, or already taking HRT, should be informed of the increase in breast cancer risk with type and duration of HRT.
Advice to individuals on the use of HRT should vary according to the individual clinical circumstances (such as asymptomatic menopausal symptoms, age, severity of menopausal symptoms, or osteoporosis).
HRT usage when at familial risk should be restricted to as short a duration and as low a dose as possible.
If having an early (natural or artificial) menopause, patients should be informed of the risks and benefits of HRT, but generally HRT usage should be confined to patients younger than age 50 years if at moderate or high risk.
Alternatives to HRT should be considered for specific symptoms such as osteoporosis or menopausal symptoms
Consideration should be given to the type of HRT if it is being considered for use in conjunction with risk-reducing gynaecological surgery.
See CCG webpage Breastfeeding and Medicines for more information on prescribing medicine in breastfeeding patients.
Breastfeeding and HRT – Oestrogen:
Breastfeeding and Oestrogen cream or pessary
Breastfeeding and HRT – Progesterone:
Mirena® 20 micrograms/24 hours intrauterine delivery system can be used as protection from endometrial hyperplasia during oestrogen replacement therapy. See SPC for detail on length of treatment. Mirena is effective for 5 years in the indication of contraception, but 4 years for the progesterone component of HRT.
Breastfeeding and Medication – The Menopause and Breastfeeding
See CCG contraception webpage
FSRH Clinical Guideline: Contraception for Women Aged over 40 Years
People with premature ovarian insufficiency will be offered sex steroid replacement with a choice of HRT or a combined hormonal contraceptive (CHC). CHC (unless contraindicated) has the added benefit of providing contraceptive cover to people with premature ovarian insufficiency.
For people with a uterus requiring protection from endometrial hyperplasia during oestrogen replacement therapy as well as contraception, Mirena® 20 micrograms/24 hours intrauterine delivery system can be used. See SPC for detail on length of treatment. Mirena is effective for 5 years in the indication of contraception, but 4 years for the progesterone component of HRT.
For people who are perimenopausal, or menopausal, see the FSRH guidance above.
Diagnose the following without laboratory tests in otherwise healthy women or people assigned female at birth (AFAB), aged over 45 years with menopausal symptoms:
-perimenopause based on vasomotor symptoms and irregular periods
-menopause in women and people AFAB who have not had a period for at least 12 months and are not using hormonal contraception
-menopause based on symptoms in women and people AFAB without a uterus.
Follow NICE guidance for further information
Consider using a FSH test to diagnose menopause only:
-in women aged 40 to 45 years with menopausal symptoms, including a change in their menstrual cycle
-in women aged under 40 years in whom menopause is suspected
Information and advice
-explain the stages of menopause
-common symptoms and diagnosis
-lifestyle changes and interventions that could help general health and wellbeing
-benefits and risks of treatments
-long-term health implications of menopause.
Education
Explain as well as changes in menstrual cycle, other symptoms may include:
-vasomotor symptoms (for example, hot flushes and sweats)
-musculoskeletal symptoms (for example, joint and muscle pain)
-effects on mood (for example, low mood)
-urogenital symptoms (for example, vaginal dryness)
-sexual difficulties (for example, low sexual desire).
Information on treatments
-hormonal, for example hormone replacement therapy (HRT). See formulary and above for further information.
-non-hormonal, for example clonidine
-non-pharmaceutical, for example cognitive behavioural therapy (CBT).
– Give information on contraception
Diagnose premature ovarian insufficiency in women aged under 40 years based on:
- menopausal symptoms, including no or infrequent periods (taking into account whether the woman has a uterus) and
- elevated FSH levels on 2 blood samples taken 4–6 weeks apart.
If there is doubt about the diagnosis of premature ovarian insufficiency, refer the woman to a specialist with expertise in menopause or reproductive medicine.
Do not diagnose premature ovarian insufficiency on the basis of a single blood test.
Do not routinely use anti-Müllerian hormone testing to diagnose premature ovarian insufficiency.
NICE Clinical Knowledge Summary: Diagnosis of premature ovarian insufficiency
See below for information on managing premature ovarian insufficiency.
Offer sex steroid replacement with a choice of HRT or a combined hormonal contraceptive to women with premature ovarian insufficiency, unless contraindicated (for example, in women with hormone-sensitive cancer).
Give women with premature ovarian insufficiency and contraindications to hormonal treatments advice, including on bone and cardiovascular health, and symptom management.
Consider referring women with premature ovarian insufficiency to healthcare professionals who have the relevant experience to help them manage all aspects of physical and psychosocial health related to their condition.
Explain to women with premature ovarian insufficiency:
- the importance of starting hormonal treatment either with HRT or a combined hormonal contraceptive and continuing treatment until at least the age of natural menopause (unless contraindicated)
- that the baseline population risk of diseases such as breast cancer and cardiovascular disease increases with age and is very low in women aged under 40
- that HRT may have a beneficial effect on blood pressure when compared with a combined oral contraceptive
- that both HRT and combined oral contraceptives offer bone protection
- that HRT is not a contraceptive.
NICE Clinical Knowledge Summary: Osteoporosis – Prevention of fragility fractures: Assessment
Review patients with premature ovarian insufficiency for risk of osteoporosis and fragility fractures. Check FRAX score and consider bisphosphonate treatment if indicated. See formulary chapter 6.6.
Ensure patients are taking vitamin D as self-care in line with [PH56] Vitamin D: supplement use in specific population groups and ensure adequate dietary intake of calcium.
The Equality Act 2010 gives legal protection to people who reassign their gender away from the sex they were assigned at birth. Trans people legally are to be treated in accordance with their legal gender identity. The Equality Act describes gender reassignment as those who ‘propose to undergo, are undergoing or have undergone a process or a part of a process’ to reassign their gender away from the sex assigned at birth.
Not all Trans people will have surgery, not all Trans people will be under the care of the Gender identity clinic (GIC) and not all Trans people will be taking hormones to affirm their gender.
The Gender Recognition Act 2005 provides Trans people with the option to apply for a Gender Recognition Certificate (GRC). This enables a person to legally change the sex detailed on their birth certificate and other legal documents. It is not a requirement to change the sex recorded on other records, such as NHS records, bank accounts, etc. Not all Trans people will choose to apply for a GRC. A GRC is not required in order to live and be afforded the protection under the Equality Act 2010, as detailed above. It is neither appropriate nor lawful to ask a person if they have a GRC.
Gender Identity Research and Education Society has information on terminology which you may find useful and more in depth than this short section.
For the purposes of the menopause page, we include women and people assigned female at birth (AFAB) who are perimenopausal or menopausal.
For more resources, please see the Equality and Diversity page.
As of 1st April 2023, a new NHS hormone replacement therapy prescription pre-payment certificate (HRT PPC) has been introduced.
Before purchasing a HRT PPC, patients should check if they are entitled to free NHS prescriptions using the eligibility checker.
The HRT PPC covers certain HRT medicines, regardless of why they are prescribed, for twice the single prescription charge and is valid for 12 months. It will save the patient money if they need to pay for 3 or more HRT prescription charges within 12 months. If patients get prescriptions for items other than HRT medicines, they may save more with a 3 or 12 month PPC that covers all NHS prescriptions.
The HRT PPC does not cover all HRT medicines. A list of eligible HRT medicines can be found on the NHSBSA website.
Due to the potential complexities which may arise when a patient only has a HRT PPC but is prescribed another medication, all prescribers are requested to ensure that HRT prescriptions have no other medication prescribed on the same form. For example, a patient has HRT and an urgent antibiotic – please ensure that two separate prescriptions are issued, a patient has HRT and a repeat statin – please ensure that two separate prescription forms are issued, etc.
If prescriptions have mixed HRT and another item on the same form but the patient only has a HRT PPC then the pharmacy may refuse to dispense some or all of the items and refer patient back to the prescriber, which would obviously cause inconvenience and additional workload to all.
N.B. Patients over 60 will be automatically exempt so can have multiple items on same prescription as HRT.
For further information see the Department of Health and Social Care (DHSC) Guidance on the Hormone Replacement Therapy prescription prepayment certificate: Handling NHS HRT prescriptions.
PSNC have produced some guidance and FAQs to support pharmacy teams in implementing the DHSC guidance.
Give advice on bone health and discuss these issues at review appointments.
Explain that the baseline population risk of fragility fracture around menopausal age in the UK is low and varies from one female to another.
Explain that their risk of fragility fracture is decreased while taking HRT and that this benefit:
- is maintained during treatment but decreases once treatment stops
- may continue for longer in women who take HRT for longer.
Review patients for risk of osteoporosis and fragility fractures in line with NICE Clinical Knowledge Summary: Osteoporosis – Prevention of fragility fractures: Assessment. Check FRAX score and consider bisphosphonate treatment if indicated. See formulary chapter 6.6 and the joint NHS Somerset Formulary.
Ensure patients are taking vitamin D as self-care in line with [PH56] Vitamin D: supplement use in specific population groups and ensure adequate dietary intake of calcium through a food first approach.
Where a patient is deficient in vitamin D (<25nmol/l serum 12-hydroxyvitamin D) a one off acute prescription should be prescribed for an appropriate loading dose before the patient returns to self-care vitamin D supplementation over the counter. Information can be found on the joint NHS Somerset Formulary.
Calcium and vitamin D supplements are not indicated for prescribing in menopausal patients unless they are prescribed bone-sparing agents.
Information should be provided on checking calcium intake through diet, using an appropriate calcium calculator, as well as information on weight bearing exercises to maintain bone health.
Useful resources:
More information can be found on the Self Care - NHS Somerset ICB page.
Consider HRT for low mood associated with menopause.
Consider CBT for low mood or anxiety associated with menopause.
There is no clear evidence for SSRIs or SNRIs.
See our mental health prescribing page for further mental health resources.
Explain to women and people with a uterus that unscheduled vaginal bleeding is a common side effect of HRT within the first 3 months of treatment but should be reported at the 3-month review appointment, or promptly if it occurs after the first 3 months
Offer women and people who are stopping HRT a choice of gradually reducing or immediately stopping treatment.
Explain that:
-gradually reducing HRT may limit recurrence of symptoms in the short term
-gradually reducing or immediately stopping HRT makes no difference to their symptoms in the longer term.
NICE indicates the use of testosterone supplementation for menopausal women with low sexual desire if HRT alone is not effective. Currently this is an off-licence indication. PAMM approved the use of testosterone within guidelines in October 2021, agreeing the Traffic Light Status as GREEN.
Testosterone can be initiated by a GP with additional training on prescribing testosterone, e.g. via BMS, CoSRH resources or local training. The Somerset NHS Menopause service can also provide advice and guidance on optimising HRT and testosterone in this group.
For detailed information see British Menopause Society – Tools for Clinicians: Testosterone replacement in menopause
BMS Statement on Testosterone - British Menopause Society July 2024
BMS Testosterone Patient Information Leaflet
Choices
First line– Testogel sachets 2.5g sachets contain 40.5mg testosterone.
Testogel sachets should last at least 8 days and will need to be prescribed with instructions:
Apply a small pea sized amount once daily to lower abdomen, buttock or outer thigh. Rotate site of application daily. Use at the same time each day. One 2.5g sachet should last at least 8 days, seal with a clip between uses.
Second line– Tostran pump (2% testosterone gel) – (60g metered dose pump) Apply one metered dose (10mg) three times a week or on alternate days to lower abdomen, buttock, or outer thigh. Rotate site of application daily. Use at the same time each day. 60g pump container delivers 120 metered doses, each cannister should last 240 days. Check stock availability before prescribing.
Do not prescribe the pump version of Testogel or the Testavan Pump as they are unsuitable for HRT in menopause
PRACTICAL TIPS ON PRESCRIBING AND USING TESTOSTERONE
- Testosterone gel should be applied to clean, dry skin of the lower abdomen, buttock, or outer thigh, rotating the site of application to avoid hair growth in one area. It should be allowed to dry before dressing. Skin contact with partners and children should be avoided until dry and hands should be washed immediately after application. The area should not be washed for 3 hours after application.
- The patient should be on transdermal estrogen at a sufficient dose to relieve most symptoms before considering if testosterone supplementation is needed.
- A blood test for testosterone level is needed before starting testosterone. This is not to make a diagnosis but to check that the patients testosterone level is not towards or above the top of the female reference range. If the level is 1.95nmol/L or above, testosterone supplementation is not indicated. (This applies to Somerset lab ranges as of October 2025- other areas have different ranges and is subject to change depending on current testing methods.)
- Blood tests are also needed 2 months after initiation or any dose change and then at least annually once stable. This is to make sure the levels are still within the female reference range.
- It can take at least 3 months to notice any difference in symptoms. If no improvement after 6 months of use, then we would suggest stopping it.
- Common side-effects when starting testosterone are greasier hair and skin, spots, increased irritability, hair thinning and weight gain. If these symptoms don’t settle the dose can be reduced before the 3-month review (but patients must never increase the dose without medical advice).
- Serious and possibly irreversible side-effects can develop if the dose is too high. These include male pattern hair loss, deepening of the voice, increased body and facial hair, and very rarely an enlarged clitoris.
- Please warn patients that the patient information leaflet only relates to male use and give them the patient information leaflet from either the British Menopause Society or Women’s Health Concern.
Patient information
With Testogel sachets it can take a while for the patient to work out how much to apply each day – suggest starting with a (small) pea-sized amount and then they can adjust up or down depending on how long the first tube/sachet lasts.
The gel should be applied to clean, dry skin and allowed to dry before dressing. Skin contact with partners and children should be avoided until dry and hands should be washed immediately after application. The area should not be washed for 3 hours after application.
Please warn the patients that the patient information leaflet only relates to male use and give them the BMS information leaflet as above.
BLOOD TESTS
A blood test for testosterone level is needed before starting testosterone treatment.
If testosterone gel is normally applied in the morning, the dose that day should be omitted until after the blood test.
Please make sure the patient has the blood results at her appointment if not available at the time of the referral.
Calculating the Free Androgen Index is no longer considered helpful.
The latest guidelines on the Management of Unscheduled Bleeding from the British Menopause Service provides a comprehensive guide to support your patients while they're taking HRT, there is also guidance on optimising progestogen dosing to protect the endometrium which has been reviewed at the Medicines Programme Board May 2024 and approved for use in Somerset.
BMS GUIDELINE Management of unscheduled bleeding HRT JULY 2024 links to the BMS website to access the document.
The BMS have produced a frequently asked questions document for the management of unscheduled bleeding December 2024.
*Content below is under review and will be updated in due course. June 2024*
Explain to women and people with a uterus that unscheduled vaginal bleeding is a common side effect of HRT within the first 3 months of treatment but should be reported at the 3-month review appointment, or promptly if it occurs after the first 3 months (see recommendations on endometrial cancer in the NICE guideline on suspected cancer).
Unscheduled bleeding after 6 months of starting HRT should be reviewed by a GP, with examination where suitable.
Patients may be reluctant to stop HRT to see if bleeding settles due to the benefits of treatment for menopause symptoms which previously affected quality of life.
Topical oestrogen may be useful in patients with vaginal atrophy, see formulary guidance below on vaginal atrophy.
Vaginal bleeding problems– A detailed history:
- Unscheduled vaginal bleeding is a common adverse effect of HRT within the first 3 months of treatment.
- Monthly cyclical regimens should produce regular withdrawal bleeding towards the end of the progestogen phase.
- Continuous combined HRT commonly produces irregular breakthrough bleeding or spotting in the first 4–6 months of treatment. If bleeding persists beyond 6 months, becomes heavier, or occurs after a spell of amenorrhoea, endometrial pathology should be excluded. See the CKS topic on Gynaecological cancers – recognition and referral for more information.
- Unpredictable or unexpected bleeding may also be due to non-adherence with treatment, drug interactions, or a gastrointestinal disorder (which may affect drug absorption).
- If serious gynaecological pathology has been excluded, altering the progestogen part of the regimen may improve bleeding problems. Options include:
Hormone replacement therapy (HRT) | Prescribing information | Menopause | CKS | NICE
Lichen sclerosus is a chronic inflammatory skin condition which can affect any part of the skin, but it most often affects the genital skin (vulva) and the skin around the anus. It can start in childhood or adulthood, most commonly after menopause. For more information see the NHS Somerset Dermatology formulary page.
For more information, see:
Hormone replacement therapy (HRT) | Prescribing information | Menopause | CKS | NICE
Recommendations | Menopause: diagnosis and management | Guidance | NICE
Gynaecological cancers – recognition and referral | Health topics A to Z | CKS | NICE
For detailed information on vulval and urinary symptoms that often develop with low estrogen levels, known as urogenital atrophy, or genitourinary syndrome of the menopause (GSM) please see the Local Estrogen - NHS Somerset ICB page.
See the Antimicrobial - NHS Somerset ICB guidance for information on the management of recurrent UTI's.
Urogenital Symptoms:
•Painful intercourse
•Vaginal bleeding – Must be investigated
•Vulvo-vaginal Discomfort
•Infection and discharge
•Itch – the skin around the vulva is more sensitive and more likely to itch in some women. This produces a tendency to scratch which then makes the skin more likely to itch. An itch/scratch cycle follows which can be both difficult to break and quite distressing.
•Urinary problems (frequency/urgency to pass urine)
Urinary symptoms that may occur include one or more of the following: –
Passing water too often (frequency)
Not being able to hold on (urgency)
Pain when passing urine (dysuria)
See formulary chapter 7.4.2 for more information on drugs for urinary frequency.
INVESTIGATE unexplained bleeding
People suffering from vaginal dryness can use genital moisturisers and lubricants, these can be used alone or in addition to local vaginal estrogen. Genital moisturisers and lubricants are suitable for self-care. People should be made aware that barrier methods for contraception may be damaged by local estrogen preparations.
Offer HRT for vasomotor symptoms after discussing with the patient the short-term (up to 5 years) and longer-term benefits and risks.
Offer a choice of preparations:
- oestrogen and progestogen to women with a uterus
- oestrogen alone to women without a uterus.
There is some evidence that isoflavones or black cohosh may relieve vasomotor symptoms (suitable for self-care).
NOTE: Health food supplements may vary, their safety isn’t regulated as medicines are and interactions may be possible.
Healthcare professionals are reminded to be vigilant for suspected adverse reactions associated with the use of herbal and homeopathic medicines and interactions with other medicines and report suspicions to the MHRA’s Yellow Card scheme.
Do not routinely offer selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs) or clonidine as first-line treatment for vasomotor symptoms alone. Clonidine is licensed for and may be considered for hot flushes in people where HRT is not suitable or compatible. Consideration should be taken for side effects and review efficacy after initiation.
Menopause and work: why it’s so important
CIPD provides printable resources to help break the stigma around the menopause in workplaces: The menopause at work: printable resources